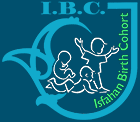Phase 2 Data collection design
In Phase 2, we will follow pilot study participants and Phase 1 general and omics cohorts:
A. General cohort:
for which we will collect data on:
- Physical examination (child)
- Anthropometric measures
- Height, weight, and head circumference: every two months from log-book
- Skinfold thickness (triceps and sub-scapular), mid-upper arm circumference, waist circumference, and hip circumference: three times at 6, 12, and 24 months
- Motor development (WHO milestone questionnaire)
- Blood pressure (twice at 12 and 24 months)
- Auditory test (once at 6 month)
- Anthropometric measures
- Questionnaires (child)
- Child
- Infections (three times at 6, 12, and 24 months)
- Time-activity (twice at 12 and 24 months)
- Asthma and wheeze (6, 12, and 24 months)
- Allergy, eczema, and food allergy (6, 12, and 24 months)
- Diet (breastfeeding, multivitamin/supplements, etc.) (three times at 6, 12, and 24 months)
- Medication (three times at 6, 12, and 24 months)
- Hospital admissions (three times at 6, 12, and 24 months)
- Passive smoking (three times at 6, 12, and 24 months)
- Neonatal jaundice (Once at 6 months)
- Mother
- Mental health (depression, anxiety, life events)
- Occupation/employment (mother and father) (Once at 12 months)
- Home move (three times at 6, 12, and 24 months)
- Home characteristics: presence of pets and animals (twice at 6 and 24 months)
- Antibiotic use (three times at 6, 12, and 24 months)
- Noise (Twice at 6 and 24 months)
- Change in marital status (three times at 6, 12, and 24 months)
- Child
- Health care and clinical data (child)
- Vaccination history: every two months from the log-book
- Growth curve: every two months from the log-book
- Biological samples
- Child
- Urine (twice at 6 and 24 months)
- Dried blood spot
B. Omics sub-cohort:
- Physical examination (child)
- Anthropometric measures: Skinfold thickness (triceps and sub-scapular), mid-upper arm circumference, waist circumference, and hip circumference (an extra time at 2 months)
- Eye refraction (twice at 12 and 24 months)
- Anogenital distance (Once at 2 months)
- Questionnaires
- Child
- FFQ (three time at 6, 12, and 24 months)
- Pacifier use/thumb sucking (6, 12, and 24 months)
- Household pesticide use (6 and 24 months)
- BPA/Phthalates
- Sleep disturbance (6, 12, and 24 months)
- RF/ELF exposure (twice at 6 and 24 months)
- Mother
- Child and mother relationship (once at 6 months)
- Social support (once at 6 months)
- RF/ELF exposure(twice at 6 and 24 months)
- Sleep disturbance (three times at 6 and 24 months)
- Contraceptive use (twice at 12 and 24 months)
- Menstruation (twice at 12 and 24 months)
- Father
- Mental health (depression, anxiety, life events)
- Sleep
- Child
- Clinical tests
- Structural MRI and resting state fMRI and Diffusion tensor imaging (DTI) MRI for a subset of 100 children
- Biological samples
- Child
- Blood (once at 12 months)
- Urine (four times at 2, 6, 12, and 24 months)
- Faeces (four times at 2, 6, 12, and 24 months)
- Hair (four times at 2, 6, 12, and 24 months)
- Nail (three times at 6, 12, and 24 months)
- Skin swab (12 months)
- Saliva (12 months)
- Mother
- Faeces (12 months)
- Skin swab (12 months)
- Saliva (6 and 12 months)
- Breast milk (two times at 2 and 6 months)
- Child
- Living environment
- Settled dust (Twice at 2 and 24 months)
Phase 2 data collection methods
We will collect data from the mother, father, and the child using questionnaires, biological samples, physical examinations, clinical tests, hospital records, and survey of the living environment as detailed in table 7. The tasks planned for each Phase 2 visit are detailed in Table 8. Table 9 describes the information planned to be collected through questionnaires.
Table 7. Data collection methods and timeline during Phase 2.
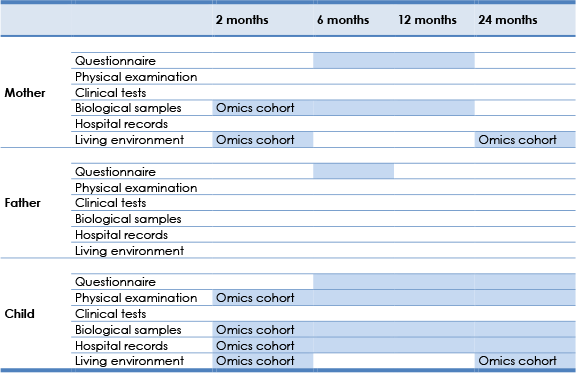
Table 8. Tasks during each Phase 2 visits. Tasks with underlined fonts apply to both father and mother, those in bold fonts apply to child, and those in normal fonts only apply to mothers. (Tasks in bold fonts are carried out only for Omics Cohort)